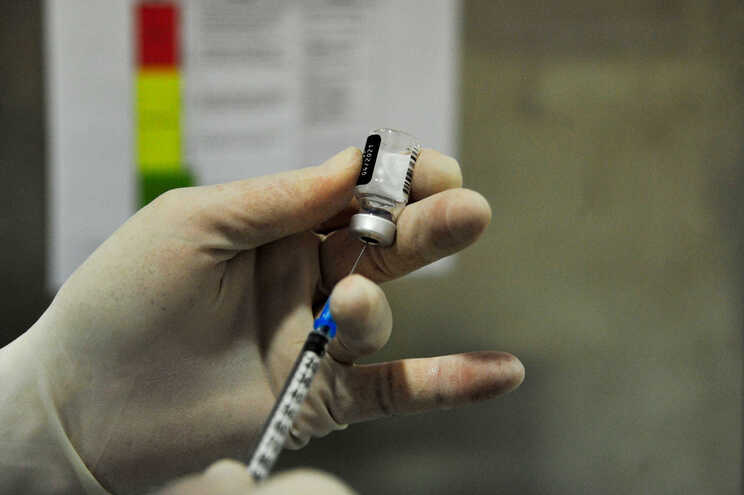
Sinopharm and subsidiary Beijing Biological Products Institute said on Wednesday that the vaccine’s effectiveness is 79.34%, according to provisional data from phase 3 clinical trials.
Chinese authorities gave the green light this Thursday for the marketing of one of the vaccines against covid-19 developed in the country, by Sinopharm and the subsidiary Institute of Biological Products of Beijing.
Both companies asked the Chinese regulatory authority on Wednesday for approval of the vaccine, after informing that the effectiveness is 79.34%, according to provisional data from phase 3 clinical trials.
In a press conference held this Thursday in Beijing, the number two regulator, Chen Shifei, explained that the institution concluded that “the known and potential benefits of this vaccine outweigh the known and potential risks” and that it complies with the rules established for the conditional approval of marketing.
Read more in Diário de Notícias